The user-friendly guide to coastal planktonic ciliates
Methods
Overview
Planktonic ciliates are microplankton (20 - 200 µm in size; Sieburth et al. 1978). The most common methods for sampling,
fixing, and staining these organisms are briefly described and/or referenced on this page. In the data sheets we include pictures obtained using these techniques.
Inverted microscopes are commonly used to quantify and identify ciliates and other microplankton in plankton samples.
Unfortunately, with routine preservation methods (e.g. Lugol’s iodine), identification of ciliates is often difficult. The
specialised technique of silver-staining, with protargol, reveals diagnostic features of ciliates and is normally used for
taxonomic descriptions. However, protargol staining is elaborate and time consuming. Therefore, it is rarely used in routine
ecological studies (Montagnes & Lynn 1993).
Plankton samples preserved with Lugol's (2 to 10 %) have low loss of cell numbers, but it is important to note that this fixative shrinks cells. In the data sheets, the average biovolume provided for taxa is based on Lugol's fixed material. A conversion factor commonly used for aloricate ciliates is 190 fg C µm-3, when the cells are preserved with 2 % acid Lugol's solution (Putt & Stoecker 1989). For other concentrations or fixatives, this factor may need to be adjusted (Jerome et al. 1993; Stoecker et al. 1994). For further suggestions on how to measure cell volumes see http://www.uvm.edu/~dkirscht/biovol.html.
Additional information on staining, fixation and carbon conversions can be obtained from manuals for microzooplankton sampling, fixation, and staining, listed in the references at the end of this page (e.g. Gifford & Caron 2000).
Plankton sampling, storage, and enumeration
Large volumes of seawater can be collected in routine, oceanographic sampling bottles (e.g. Niskin bottles). Immediately after sampling, subsamples of a few millilitres to several litres can be taken and preserved with Lugol’s iodine (see below). Sampling with bottles minimises the loss of cells and cell disruption due to the turbulence and pressure caused by pumps and plankton nets (Gifford & Caron 2000).
After Lugol’s fixed samples have been collected, they can be stored in a cool, dark place for weeks. NOTE - there has been little evaluation of the effects of extended storage (Gifford and Caron 2000). Ciliate abundance can be obtained by settling the fixed samples in settling chambers and examining them under an inverted microscope (Hasle 1978, Gifford & Caron 2000).
Two sources for settling chambers used for inverted microscopy are:http://www.duncanandassociates.co.uk
Duncan & Associates, Jeeves Bank, Fernleigh Road, Grange-over-Sands, Cumbria LA11 7HT, UK
Tel. +44 15395 33857, Fax: +44 15395 34963
http://www.kc-denmark.dk
KC Denmark - Research Equipment, Holmblads-vej 19 - DK-8600 Silkeborg - Denmark
Tel. +45 86828347, Fax +45 86824950
E-mail: kc@kc-denmark.dk
Lugol's fixation
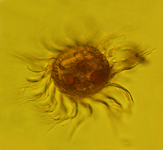
Microplankton samples should be fixed immediately to avoid loss of cells. Samples should also always be rapidly added to the concentrated fixative, diluting them to the final concentration. For our samples we used a final concentration of 2 to 5 % Lugol's iodine (vol:vol).
Acid Lugol's solution (Throndsen 1978):
Dissolve 100 g KI in 1 L of distilled water. Dissolve 50 g iodine (crystalline) in 100 ml glacial acetic acid. Mix these two
solutions. Remove any precipitates. Store in the dark.
Advantages and disadvantages of Lugol’s:
Lugol’s acts as a fixative-preservative-stain and is better for accurately quantifying ciliates than many aldehyde-based
fixatives (Stoecker et al. 1994; Throndsen 1978). However, Lugol’s masks chlorophyll fluorescence, which may be needed to
recognise mixotrophic species (Gifford & Caron 2000). Furthermore, Lugol’s will dissolve hard structures such as
coccoliths and diatom frustules and, therefore, is not ideal for long-term storage of many plankton taxa (Gifford & Caron
2000).
Lugol's solution is also iodine-based and is relatively harmless compared to aldehyde-based or other more toxic fixatives.
Furthermore, iodine enhances the sinking of cells in settling chambers. Lugol's not only fixes cells but also stains them a dark
brown colour. This simplifies counting but obscures some of the characteristic features of the ciliates (e.g. macronucleus).
Darkly stained specimens can be cleared with sodium thiosulphate (see DAPI staining below).
Lugol’s does not necessarily preserve the cell shape and size of live specimens. Thus, comparison of live and Lugol’s
fixed material is not always possible. However, Lugol's fixed material can be processed in several ways: SEM (Montagnes &
Taylor 1994), DAPI (see below), protargol staining (Montagnes & Lynn 1993). Thus, Lugol’s is a relatively harmless and
versatile fixation method, which we recommend for routine sampling of planktonic ciliates.
DAPI staining of Lugol's fixed samples

DAPI is a fluorescent dye that binds to nuclear DNA. The fluorochrome is excited by UV-light and can be observed with epifluorescence microscopy, using an inverted microscope. In conjunction with E. Lessard (Oceanography, Univ. Washington) we are developing a technique to stain Lugol’s fixed plankton samples with DAPI. This allows the nuclear shape of microplankton to be used as a diagnostic feature in routine plankton analysis. This method is being prepared for publication (Strüder-Kypke et al. in prep); we provide a preliminary protocol below.
DAPI protocol:
1. Prepare a DAPI (4',6-diamino-2-phenylindole dihydrochloride) stock solution of 150 µg ml-1.
2. Dilute this stock solution with distilled water to a DAPI working solution of 15 µg ml-1. Both the stock and
the working solution should be stored in the dark at ~ 4 ºC.
3. Settle 2 % Lugol’s fixed material in a 10 ml settling chamber.
4. With a pipette, add a small amount of saturated sodium thiosulphate (150-200 µl) to the sample. Sodium thiosulphate
bleaches the sample, reducing the darkness of the Lugol’s fixed cells and enhancing the brightness of the fluorescence.
The quantity of sodium thiosulphate can be varied to achieve the desired effect. NOTE - using large amounts of sodium
thiosulphate can bleach the organisms too much; they become colourless and difficult to find.
5. With a micropipette, add 150 µl of the DAPI working solution to the sample (final concentration: 2.25 µg per
10 ml). At this concentration, DAPI should stain the nuclei. If higher concentrations are used, DAPI may stain the entire
cytoplasm.
6. Incubate the sample for 3 - 5 min in the dark.
7. The sample can be observed with epifluorescence microscopy, using an UV filter (excitation 365 nm) The stained nucleus will
fluoresce bright blue. NOTE - Samples stained with DAPI should be kept away from bright light, as DAPI is light sensitive and
fades quickly.
Protargol staining of Lugol's fixed samples
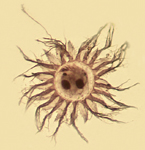
Silver staining with protargol (silver-proteinate) reveals diagnostic features of ciliates (e.g. the nuclei and infraciliature of both the cell body and oral regions). Through a series of steps, the silver-proteinate is preferentially bound to these structures. Then, the silver is "developed", much like the process of black & white photography, and the structures are revealed. Protargol is routinely used for taxonomic descriptions, but can also be applied to ecological studies if a detailed knowledge of taxa is required. For more information about protargol staining, see Montagnes & Lynn (1993, 1987) or the review from Foissner (1991).
Two protocols are recommended for protargol staining of Lugol's fixed samples. The first protocol, after Wilbert (1975), involves manipulation of single cells. The cells are stained in embryological dishes and the liquids are removed with micropipettes. This requires some practice but provides good stains, as it allows you to watch the developing process. The second method is quantitative (QPS - Quantitative Protargol Staining: Montagnes & Lynn 1993, 1987). In this method, the cells are concentrated on filters and embedded in agar. All steps are then performed in small staining jars. Using QPS, the ciliates of a subsample can be identified to the species level and counted; thus species abundance can be determined. Unlike Lugol’s, protargol has the added benefit of providing a permanent record.
NOTE - Lugol’s fixed samples must be postfixed with concentrated Bouin’s (5 % final concentration) before they can be processed (Montagnes & Lynn 1993).
SEM (Scanning Electron Microscopy)
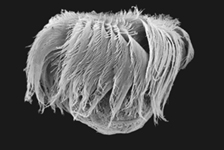
Scanning electron microscopy images provide a three dimensional image of the ciliate and its surface structures. However, ciliate species cannot be adequately identified by scanning electron microscopy alone, as several diagnostic features are not revealed (e.g. nuclear shape and number, details of the cytopharyngeal apparatus). The cilia are normally retained and therefore the ciliation is shown clearly. Heavily ciliated cells can be deciliated (for description of the method cf. Jakobsen & Montagnes 1999) to reveal otherwise hidden features (e.g. course of the kineties, number of kinetosomes, short cilia in specialised regions like the brosse in prostomes or didiniids).
The protocol can be performed using Lugol’s fixed material. The fixed cells are dehydrated in an ethanol series, critical point dried and mounted on aluminium stubs for SEM. Subsequently, they are gold coated (25 nm) and finally viewed with an SEM (Montagnes & Taylor 1994). Several other protocols for this procedure have been described (cf. Foissner Berger & Schaumburg 1999; Jakobsen & Montagnes 1999; Jonsson 1987; McManus & Fuhrman 1986).
References
Foissner W (1991) Basic light and scanning electron microscope methods for taxonomic studies of ciliated protozoa. Europ J Protistol 27: 313-330
Foissner W, Berger H, Schaumburg J (1999) Identification and ecology of limnetic plankton ciliates. Informationsber. des Bayer. Landesamtes für Wasserwirtschaft, Heft 3/99
Gifford DJ, Caron DA (2000) Sampling, preservation, enumeration and biomass of marine protozooplankton. In: Harris RP et al. (eds) ICES Zooplankton methodology manual. Academic Press, London, p 193-221
Hasle GR (1978) The inverted microscope method. In: Sournia A (ed) Phytoplankton manual. UNESCO Paris, p 88-96
Harris RP, Wiebe PH, Lenz J, Skjoldal HR, Huntley M (2000) ICES Zooplankton methodology manual. Academic Press, London
Jakobsen HH, Montagnes DJS (1999) A redescription of Balanion comatum Wulff, 1919 (Prorodontida, Ciliophora), with notes on its cultivation and behaviour. J Euk Microbiol 46:198-205
Jerome CA, Montagnes DJS, Taylor FJR (1993) The effect of quantitative protargol stain and Lugol’s and Bouin’s fixatives on cell size: a more accurate estimate of ciliate species biomass. J Euk Microbiol 40: 254-259
Jonsson, PR (1987) Photosynthetic assimilation of inorganic carbon in marine oligotrich ciliates (Ciliophora, Oligotrichina). Mar Microb Food Webs 2:55-68
Kemp PF, Sherr BF, Sherr EB, Cole JJ (1993) Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton
McManus GB, Fuhrman JA (1986) Photosynthetic pigments in the ciliate Laboea strobila from Long Island Sound, USA. J Plankt Res 8:317-327
Montagnes DJS, Lynn DH (1987) A quantitative protargol stain (QPS) for ciliates: method description and test of its quantitative nature. Mar Microb Food Webs 2: 83-93
Montagnes DJS, Lynn DH (1993) A quantitative protargol stain (QPS) for ciliates and other protists. In: Kemp PF et al. (eds) Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, p 229-240
Montagnes DJS, Taylor FJR (1994) The salient features of five marine ciliates in the class Spirotrichea (Oligotrichia), with notes on their culturing and behaviour. J Euk Microbiol 41: 569-586
Putt M, Stoecker DK (1989) An experimentally determined carbon : volume ratio for marine ‘oligotrichous’ ciliates from estuarine and coastal waters. Limnol Oceanogr 34: 1097-1103
Sieburth JMcN, Smetacek V, Lenz J (1978) Pelagic ecosystem structure: heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnol Oceanogr 23: 1256-1263
Stoecker DK, Gifford DJ, Putt M (1994) Preservation of marine planktonic ciliates: losses and cell shrinkage during fixation. Mar Ecol Prog Ser 110: 293-299
Throndsen J (1978) Preservation and storage. In: Sournia A (ed) Phytoplankton manual. UNESCO Paris, p 69-74
Wilbert N (1975) Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos 64: 171-179
[Printversion] [Copyright] [Home]









